Aluminum is an interesting metal – light, relatively strong, and naturally corrosion resistant. Unfortunately, the natural oxide surface that forms on aluminum alloys is very thin and easily damaged. Anodizing was developed to improve the properties of that oxide layer. It is an electrochemical process that produces a relatively thick and uniform layer to enhance corrosion resistance.
In addition to improving corrosion resistance, anodizing also increases abrasion resistance. Aluminum oxide is very hard, nearly as hard as diamond. So hard, in fact, that it is used as the abrasive for some sandpaper and for sandblasting media. As the oxide layer thickness increases, so does the abrasion resistance. Thicker coatings may be advantageous for parts out in the airstream or near the ground, since they tend to get “sand blasted” by road debris or during the occasional off-road excursion.
For our applications, probably the most relevant improvement achieved through anodizing is appearance. With proper prefinishing (cleaning and polishing), special procedures, and dyes, almost any color scheme can be produced including vibrant blues, reds, yellows, and even clear. In fact, aluminum can be made to look like a number of different metals, including copper and stainless steel. The resulting surface is good looking, durable, and easy to clean.
How is the coating formed?
Unlike plating, which is a deposition process, anodizing is a conversion process. During plating, the metals are deposited on the part surface. With anodizing, the surface of the aluminum is converted to aluminum oxide through an electrochemical reaction. The parts are placed in an acid bath and electricity is used to speed up the oxidation process. The properties and thickness of the coating vary with the acid bath used and the process time.
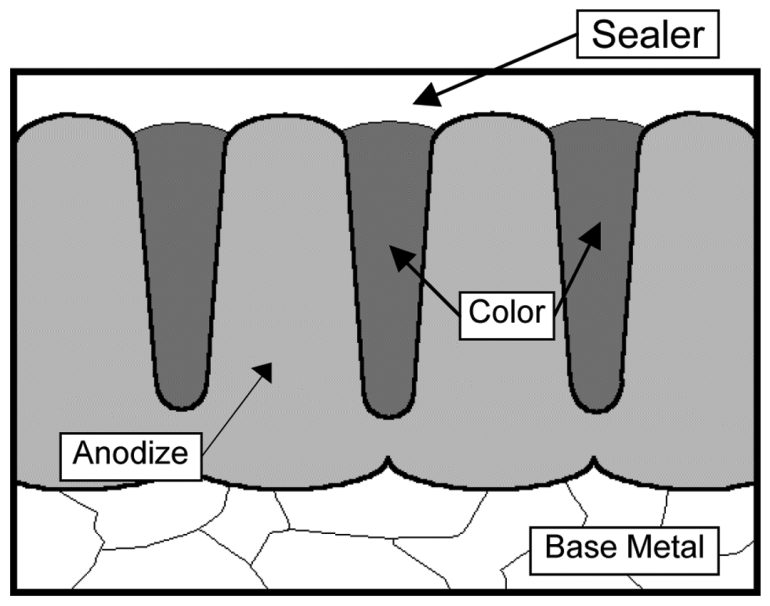
During the process, the coating formation actually builds inward, resulting in less thickness increase when compared to plating. Approximately 50% of the coating thickness is internal, meaning that a 0.001-inch thick layer will result only in a 0.0005-inch change in the part dimensions. Most of the formed oxide layer is porous, which is why the layer can be colored.
Common process types
The most common anodizing process is sulfuric acid anodize. This process produces coatings that range from 0.0001 to 0.001-inch thick and, depending on the alloy anodized, will be light yellow, gray, or tan in its natural state. The coating is usually dyed to the color of choice after oxide formation and sealed after coloring.
Multicolor splash anodizing is a relatively new variation of the sulfuric acid process and produces some interesting colors and patterns. The appearance looks similar to an air-brushed painting, but with the corrosion resistance of typical anodized coatings. If you have internet access, check out www.pkselective.com for more information.
Hard anodizing is a modification of sulfuric acid anodizing that produces a coating with thickness ranging between 0.001 and 0.003 inch. Very hard, dense, and very wear-resistant coatings are produced. This process is used primarily on parts for industrial applications, such as hydraulic cylinders and pistons to extend service life.
Chemical conversion coating, also known as chromic acid anodizing, produces a thin, corrosion-resistant oxide layer. Because the coating is thinner than produced with the other anodizing processes, it is also less abrasion resistant. Thickness normally ranges between 0.0001 and 0.0003 inch. This process is used primarily to produce a base coating for paint.
Which process to use?
Chances are, you will use sulfuric acid anodizing for your application. Most vintage racecar applications only require corrosion protection and standard abrasion resistance. If you plan on painting your aluminum parts, use a chemical conversion coating for a base coat.
Before you take the parts to the processing house, try to find out which aluminum alloy was used to make the parts. Process variables can change depending on the alloy, so you can save the processor some time if you can provide this information. If you are having parts re-anodized, it’s probably best to bring them in “as-is” and let the processor strip, polish, and clean them to meet your new requirements. If the parts are critical load-bearing components, have the processor test them for cracks using fluorescent dye penetrant inspection techniques. If you have newly fabricated parts, supply them as clean as possible. In most cases, the process house can provide the final polishing or glass beading to prep the surfaces for the look you want. Ask for a coating thickness on the high side of the range approaching 0.001 inch for maximum abrasion resistance. Select the color you want from the samples they provide or bring in a sample of something you have in mind. They may be able to provide a color that is close to your sample. Make sure that the processor seals the parts after anodizing and dyeing. This step is required to keep the colors from bleeding, to insure uniform corrosion resistance, and to prevent staining.
When assembling the newly anodized parts, use soft or padded tools to prevent dinging or scratching. With proper care and cleaning, the newly anodized parts should look as good as new for some time.